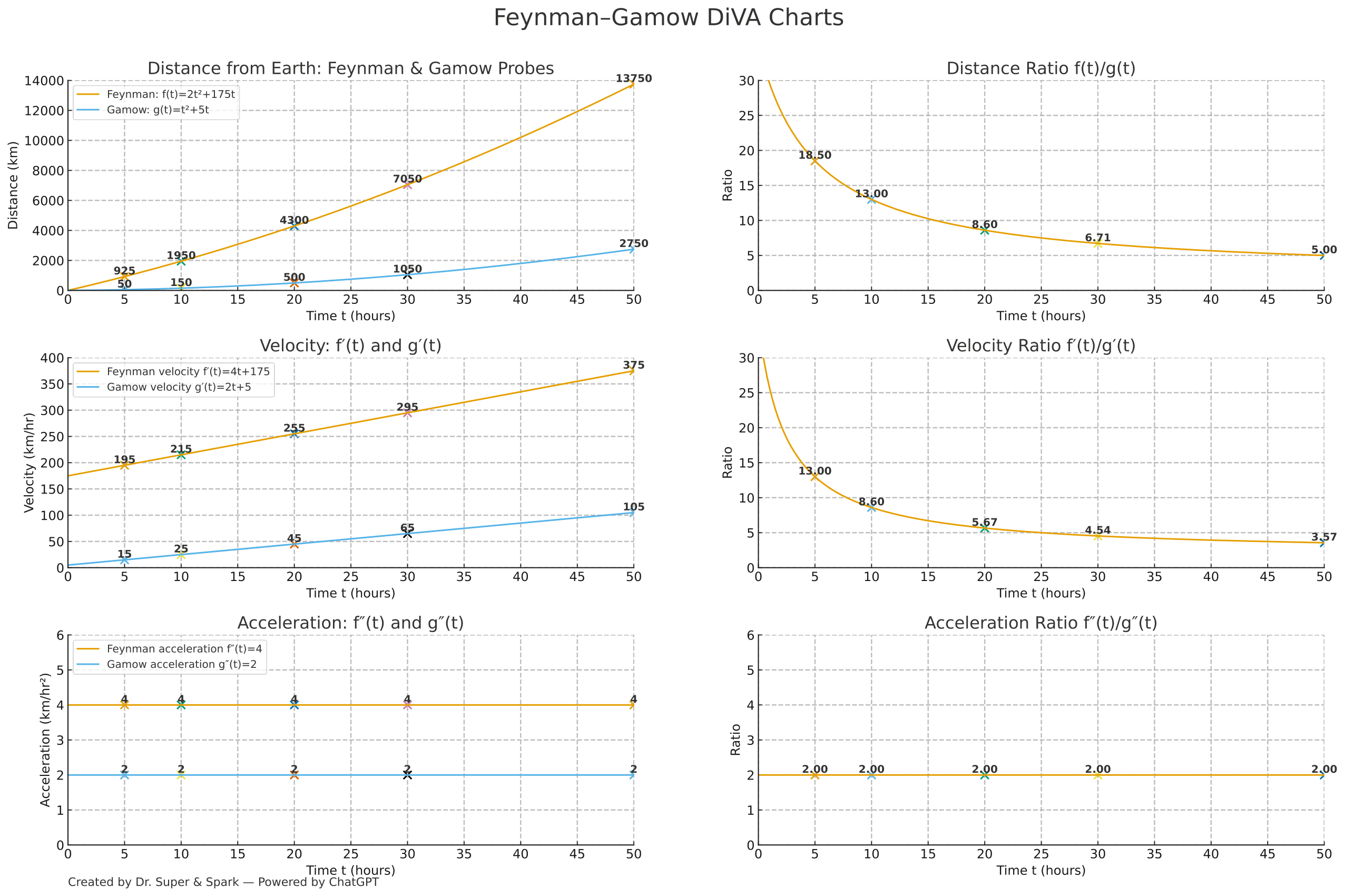
Feynman–Gamow Mission — Full Charts with Values (L, M, v, a)
Use the chart to estimate values (close estimates are fine). Units: t in billions of years, mass in grams.

We start with the exponential because it keeps its shape when you differentiate.
We model decay as M(t)=M(0)e^{-kt}. U‑235 half‑life is about 0.704 billion years.
Start with 1024 g. Each half‑life cuts the remaining mass in half.
Use the green curves for Lead L(t) (solid) and Uranium M(t) (dashed). Use the red curve for v(t).
The area under v(t) from 0 to 1 is the total mass that has changed into lead by time 1.
L(t)=1024 − M(t). If you read one, you can get the other.e^x is e^x, and d/dx of e^{g(x)} is g'(x)e^{g(x)}.k = ln(2)/T; for T=0.704, k≈0.985.L(1)≈644, L(2)≈884.